Unique System Features
-
Specifically designed for correlative light-electron microscopy (CLEM) applications
-
Capture 3D structured illumination microscopy (SIM) images at cryogenic temperatures
-
Compatible with, and independent of, later sample preparation for electron microscopy
-
Low temperature means near-zero photobleaching, producing uniform intensity 3D SIM images.
-
Widefield multi-image tiling also captures a diffraction-limited image of an entire coverslip automatically in minutes. This allows users to search for and select the best candidate cells for subsequent SIM and FIB-SEM imaging.
-
Automated SIM alignment and motorized correction collar adjustment optimizes super-resolution image quality in each field of view
System Specifications
-
Nikon 100x, 0.85 NA CFI Plan Achro LWD objective
-
Excitation Wavelengths/Powers:
-
488 nm/500 mW
-
532 nm/1000 mW
-
561 nm/500 mW
-
642 nm/500 mW
-
-
"True" 3D SIM acquisition (5 phase, 3 angle), generated via a digital spatial light modulator
-
Optimized Gustafsson SIM reconstruction algorithm
-
Sample chamber holds up to five sample coverslips at once
-
Cryostat maintains samples in vitreous state for long-term cryo-imaging (<78 degrees K), and automatically alerts users if sample temperature or pressure deviates beyond operational limits
-
Detection via Hamamatsu Orca Flash 4.0 sCMOS camera for high dynamic range and sensitivity
Limitations and Considerations
-
SIM resolution is compromised by lower system NA. This is due to the requirement that the objective image through vacuum.
-
Molecules of interest should be fluorescently tagged prior to cryo-preservation. Therefore, samples should be compatible with live-cell labeling.
-
High accuracy co-registration of optical and EM images is challenging, and requires imaging biological structures such as mitochondria that are easily visible in both modalities.
-
Segmenting objects/structures of interest from EM images is also challenging due to the large data volume. While some algorithms are available, work is ongoing to improve automation and accuracy.
Instrument Summary
The CryoSIM system is designed to generate conventional and structured illumination images at very low temperatures. Such "cryo-fluorescence" imaging has two important advantages over imaging at ambient conditions. First, fluorophore bleaching can be reduced to near-zero. This can permit users to make multiple measurements of the same cell at varying acquisition conditions to generate an "optimal" image. It also means that during 3D SIM imaging, each z-slice maintains uniform intensity, rather than decaying with distance from the coverslip as is often seen at room temperature. This makes biological interpretation easier in many cases.
More fundamentally, however, cryo-fluorescence imaging affords a unique opportunity to combine optical and EM imaging to exploit each modality's unique advantages. Such correlative light/electron microscopy (CLEM) techniques can be powerful for producing both ultrastructural and functional/molecular-specific information in the same context. However, room temperature CLEM often forces trade-offs between fluorophore retention (for better optical images) and structure preservation (for better EM images). Cryo-CLEM, on the other hand, allows for optical imaging that is independent of EM preparation/imaging. To see why, consider the figure below that summarizes a generalized cryo-CLEM workflow.

In this protocol, samples are first cultured and labeled on sapphire coverslips. Then, they are subject to a cryopreservation technique such as high-pressure freezing (HPF). At this point the samples can be placed in the cryoSIM system and imaged optically. Only after optimal super-resolution images of all regions of interest have been captured are the samples then subjected to EM processing, including freeze substitution, staining, and resin embedding. Finally samples are imaged in a suitable EM system, such as the AIC's FIB-SEM.
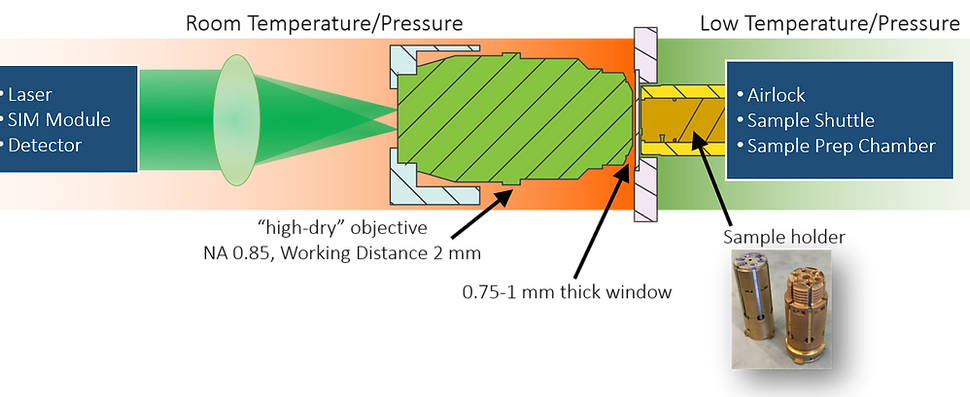
As can be surmised, however, imaging biological samples at cryogenic temperatures comes with its own set of technical challenges. Among these is the need to both (1) place the samples in a temperature controlled vacuum chamber called a cryostat while (2) maintaining the necessary illumination and detection optics at room temperature and pressure. To do this, the CryoSIM uses a customized sample holder and modified cryostat that will hold up to 5 sample coverslips at once in high vacuum and low temperature. A 1mm thick glass window is placed in front of the sample that provides a transparent barrier between the cryostat and the outside environment. Finally, a 100x Nikon NA 0.85 "high dry" objective with working distance of 2mm is used to deliver the light patterns onto the sample, and to collect the resulting fluorescence for imaging onto a Hamamatsu Orca Flash 4.0 sCMOS detector.
Two important automated features have been incorporated into this system to maximize image quality. First, optical aberrations can be especially prevalent when using "high-dry" objectives such as the one utilized here. As such, careful coverslip correction collar adjustment is vital. Our CryoSIM system features a motorized correction collar adjustment that allows users to quickly minimize aberrations due to variations in window and sapphire coverslip thickness. Secondly, alignment of the laser pattern relative the objective is critical for creating high quality SIM images. This system features an alignment algorithm that creates an image of the objective pupil plane, and automatically positions the laser illumination precisely in the center to ensure optimal light patterns on the sample.
The CryoSIM offers a uniquely advantageous tool for CLEM applications. However, as a "stand-alone" structured illumination method, is suffers from poorer resolution than what is possible at ambient conditions. This is because of the inherent limitations placed on the system numerical aperture (NA). Cryo-fluorescence imaging cannot incorporate the use of water or oil-immersion objectives, thus the lateral resolution obtainable with CryoSIM is approximately 150 nm. Potential users should therefore consider conventional SIM imaging for a standalone application without an EM component. Overall, we strongly encourage prospective AIC applicants to contact us to discuss their needs and biological questions.
Suggested Reading:
-
Hoffman, David P., Gleb Shtengel, C. Shan Xu, Kirby R. Campbell, Melanie Freeman, Lei Wang, Daniel E. Milkie et al. "Correlative three-dimensional super-resolution and block face electron microscopy of whole vitreously frozen cells." bioRxiv (2019): 773986.
-
Xu, C. Shan, Kenneth J. Hayworth, Zhiyuan Lu, Patricia Grob, Ahmed M. Hassan, Jose G. Garcia-Cerdan, Krishna K. Niyogi, Eva Nogales, Richard J. Weinberg, and Harald F. Hess. "Enhanced FIB-SEM systems for large-volume 3D imaging." Elife 6 (2017): e25916.
-
Hauser, Meghan, Michal Wojcik, Doory Kim, Morteza Mahmoudi, Wan Li, and Ke Xu. "Correlative super-resolution microscopy: new dimensions and new opportunities." Chemical reviews 117, no. 11 (2017): 7428-7456.
-
De Boer, Pascal, Jacob P. Hoogenboom, and Ben NG Giepmans. "Correlated light and electron microscopy: ultrastructure lights up!." Nature methods 12, no. 6 (2015): 503.
-
Tuijtel, Maarten W., Abraham J. Koster, Stefan Jakobs, Frank GA Faas, and Thomas H. Sharp. "Correlative cryo super-resolution light and electron microscopy on mammalian cells using fluorescent proteins." Scientific reports 9, no. 1 (2019): 1369.
